As gasoline is highly explosive,
especially when under high pressures (early Stanley Steamers that used only
gasoline for fuel required the gasoline be pressurized to 100 PSIG or more),
the use of kerosene as the fuel source for the burner was made in 1913.
However, as gasoline is easier to vaporize it was maintained for use with
the pilot. This necessitated an additional tank be added to the car to hold
the gasoline for the pilot. With the use of gasoline for the pilot it was
now possible to greatly reduce the pressures used to force the gasoline to
the pilot as well as the volume of gasoline carried on the vehicle.
As the internal combustion engine
found increased acceptance, refineries started adding chemicals to their
gasolines and making other changed to the fuel to improve combustion
performance when used with internal combustion engines. In the teens and
twenties there were also concerns about refining enough gasoline and thus
experimentation into alternate fuels such as ethyl alcohol. This led to the
distinction of some gasolines being called "white gas" due to a water white
appearance and the fact that this form of gasoline is purely refined from
crude oil with no additives. Readily available at hardware stores and gas
stations, this was the original motor fuel, and was also known as casing
head gas. White gas was often used as a cleaning agent and a fuel for
outboard motors and early powered lawnmowers.
Once the internal combustion engine
became the power source of choice for automobiles, improvements in gasoline
composition continued with all sorts of formulas being tried. Internal
combustion engine designers needed to get more power out of the engines to
power the heavier cars. One of the things they wanted to do was increase the
compression of the engines to get more energy from the fuel but this caused
a condition known as pre-ignition or "knocking". During the 1920s automotive
gasolines were undergoing constant experimentation to resolve their tendency
to pre-ignite under which caused engines to run rough. Alternate fuels such
as ethyl alcohol (ethanol) and benzene were considered as well as hundreds
of additives. Most notable of the these additives is tetraethyl lead, Pb(CH2CH3)4.
When added to gasoline, particles of lead and lead oxide PbO are formed on
combustion that helps the gasoline burn more slowly and smoothly, preventing
knocking. Today the alternative to the outlawed tetraethyl lead is an
additive called MTBE, which stands for methyl tertiary-butyl ether, and it
is designed to reduce carbon monoxide and ozone emissions as well
When the resurgence of interest in
Stanley Steam Cars arose in the 1960s and later, owners found that the
gasolines of the day had too many additives to burn cleanly. They were
difficult to vaporize and the additives tended to foul the vaporization
tubes of the pilot. White Gas was still available but even it wasn’t the
purely refined gasoline that was available when Stanley Steamers were in
general use. An alternative fuel used by campers for stoves and fuels found
acceptance as an alternative to white gas.
Coleman fuel is basically white
gasoline but it does contain components that are much less volatile than
gasoline. This is what makes it safer to use in a stove or lantern. The
Coleman fuel of today has not changed in years; it is blended of gasoline,
naphtha, xylene, toluene, and benzene. There is a rust inhibitor along
with a green dye added for identification (click here to review the
Coleman Fuel Specification using Adobe
Acrobat). An interesting piece of trivia is that Coleman Fuel is the preferred fuel
for fire-eaters!
Even the additives and blending of
Coleman Fuel have given some Stanley owners problems in the operation of
their pilots. An alternative fuel, hexane, is now being used by many Stanley
owners who can purchase it in quantity for their cars. Hexane, the common
name n-Hexane, is a very volatile aliphatic hydrocarbon. It is a
constituent in the paraffin fraction of crude oil and natural gas and is
also used as an industrial chemical and laboratory reagent.
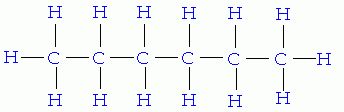 The chemical formula for hexane is C6H14. The
structure of hexane is written as CH3(CH2)4CH3
and the atoms bond together as depicted to the left. Hexane has a
faint peculiar odor that is generally considered disagreeable, it is
non-polar and thus insoluble in water. It vaporized easily as its
boiling point is 68.7° Centigrade. Of greatest importance to Stanley
owners is hexane's easy of vaporization and the fact that it is a pure fuel
and thus it burns very cleanly with little opportunity to plug up vaporizers
and the pilot itself.
The chemical formula for hexane is C6H14. The
structure of hexane is written as CH3(CH2)4CH3
and the atoms bond together as depicted to the left. Hexane has a
faint peculiar odor that is generally considered disagreeable, it is
non-polar and thus insoluble in water. It vaporized easily as its
boiling point is 68.7° Centigrade. Of greatest importance to Stanley
owners is hexane's easy of vaporization and the fact that it is a pure fuel
and thus it burns very cleanly with little opportunity to plug up vaporizers
and the pilot itself.

 The chemical formula for hexane is C6H14. The
structure of hexane is written as CH3(CH2)4CH3
and the atoms bond together as depicted to the left. Hexane has a
faint peculiar odor that is generally considered disagreeable, it is
non-polar and thus insoluble in water. It vaporized easily as its
boiling point is 68.7° Centigrade. Of greatest importance to Stanley
owners is hexane's easy of vaporization and the fact that it is a pure fuel
and thus it burns very cleanly with little opportunity to plug up vaporizers
and the pilot itself.
The chemical formula for hexane is C6H14. The
structure of hexane is written as CH3(CH2)4CH3
and the atoms bond together as depicted to the left. Hexane has a
faint peculiar odor that is generally considered disagreeable, it is
non-polar and thus insoluble in water. It vaporized easily as its
boiling point is 68.7° Centigrade. Of greatest importance to Stanley
owners is hexane's easy of vaporization and the fact that it is a pure fuel
and thus it burns very cleanly with little opportunity to plug up vaporizers
and the pilot itself.