There are many types of burners used
on Stanley steam cars. While cars were shipped from the factory with a
Stanley burner installed, many owners over the years changed to an
aftermarket burner. Such names as Cruban, Baker, and Ottaway are some of the
more common ones in use.
Stanley grates are cast iron and are
heavy and thick. Built two types, slotted and drilled, the burners were
always the same diameter as the boiler. Stanley burner grates have flat
peaks milled flat for about 1" of width. Between each of these flat peaks
were valleys about 1" wide and 1" deep. These valleys were leveled to the
height of the peaks with insulation to keep the heat off of the grate. The
slotted grate had short slots milled across the top of each ridge. The
drilled burner grate has thousands of holes drilled through the ridge to
allow the air/fuel mixture to pass. The slotted grate was used up to about
1914. After1914 the drilled grate was used with either of their two fuel
systems.
The Baker burner utilizes a thin,
slotted, flat grate casting. The long slots allow much more air/fuel mixture
through than the other types of burners. They are known to give the most
heat of any burner. Bakerís mixing tubes were nearly twice the
cross-sectional area of a pair of Stanley nozzles.
The Ottaway style burner is a
fabricated burner that is easy for hobbyists to build. It is a round
pancaked air chamber with fuel mixing tube in the front like the others. The
chambers top grate area is 5/32 " thick # 321 stainless plate. The bottom
plate is identical but without the holes obviously. The grate area is solid
(no insulation) with #54
drill bit holes laid out on a grid.
 Cruban offered a burner and pilot
light combination of their own design. Their burners were much heavier than
a Stanley being all cast construction. Cruban also built Stanley burner
bottoms using their Nichrome heat resisting lining. A Cruban grate is
similar in design to the Stanley slotted design. Crubanís aftermarket pilot
for use with a Stanley burner looked different in that they had a vertical
air intake. Click on the following link to view a series of
advertising cards describing the design features of the Cruban burner.
Also see the Steam Automatic article for information relating to the Cruban
Steam Automatic.
Cruban offered a burner and pilot
light combination of their own design. Their burners were much heavier than
a Stanley being all cast construction. Cruban also built Stanley burner
bottoms using their Nichrome heat resisting lining. A Cruban grate is
similar in design to the Stanley slotted design. Crubanís aftermarket pilot
for use with a Stanley burner looked different in that they had a vertical
air intake. Click on the following link to view a series of
advertising cards describing the design features of the Cruban burner.
Also see the Steam Automatic article for information relating to the Cruban
Steam Automatic.
~ Cruban
Empire Burner Advertising Cards ~
Pictured to the left is a Cruban
burner belonging to Bruce Magnell undergoing a test firing. This
burner has been rebuilt and is being tested prior to being put under a
boiler. During testing a specific amount of kerosene was put in a tank
and the tank pressurized to a given pressure. The burner was lit and
allowed to consume all the fuel. The fuel consumption rate of the
burner was then determined.
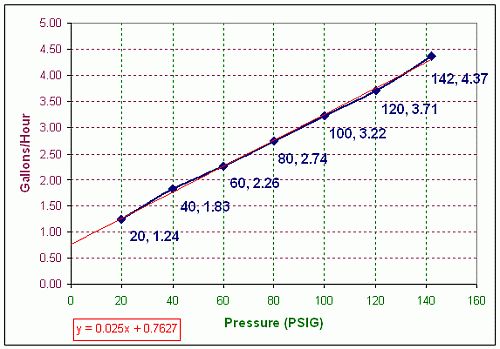
The graph below indicates now the fuel
consumption changes with the fuel's applied pressure. The blue line
represents the data obtained during the test. The red line is an Excel
best fit linear trendline for the data. The data nearly follows the
following equation: y = 0.025x + 0.7672. Data has yet to be correlated
with the size of the nozzle orifice.
 The
energy content of kerosene is between 130,000 BTUs and 135,000 BTUs per
gallon. The heat content of one gallon of kerosene roughly equals that
of 41 kilowatt-hours (kWh) of electricity, 137 cubic feet of natural gas,
1.5 gallons of propane, 17.5 pounds of air-dried wood, a gallon of diesel
fuel, or 10 pounds of coal. The Cruban burner in the experiment has
the capability to generate 500,859 BTUs per hour (135,000 BTUs per gallon of
kerosene x 3.71 gallons/hour). One (1) boiler horsepower is defined as
33,475 BTUs per hour thus the Cruban burner of the experiment is generating
about 15 boiler-horsepower (assuming total efficiency).
The
energy content of kerosene is between 130,000 BTUs and 135,000 BTUs per
gallon. The heat content of one gallon of kerosene roughly equals that
of 41 kilowatt-hours (kWh) of electricity, 137 cubic feet of natural gas,
1.5 gallons of propane, 17.5 pounds of air-dried wood, a gallon of diesel
fuel, or 10 pounds of coal. The Cruban burner in the experiment has
the capability to generate 500,859 BTUs per hour (135,000 BTUs per gallon of
kerosene x 3.71 gallons/hour). One (1) boiler horsepower is defined as
33,475 BTUs per hour thus the Cruban burner of the experiment is generating
about 15 boiler-horsepower (assuming total efficiency).


 A proper ratio of gaseous (vaporized) fuel to air is
required for combustion. Every fuel has a flammability range. Combustion is
only possible if the fuel-air ratio is within the flammability range for the
fuel. A fuelís flammability range refers to the percentage of the fuel, in
its gaseous state, to air to create a combustible or explosive mixture. This
varies with different flammable liquids. Gasoline has a flammability range
of 1.4 % to 7.6 %. This means gasoline will ignite when there is 1.4 parts
of gasoline mixed with 100 parts air. Thus, 1.4 % is known as the lower
flammable limit and 7.6 % is the upper flammable limit of the flammable
range. A product mixed with air below the low end of its flammable range is
too lean to burn. A flammable liquid that exceeds its upper flammable limit
is too rich to ignite. Ethylene oxide is extremely flammable. It has a
flammable range of 3.6 % to 100 %. This means it can burn even if there is
no air.
A proper ratio of gaseous (vaporized) fuel to air is
required for combustion. Every fuel has a flammability range. Combustion is
only possible if the fuel-air ratio is within the flammability range for the
fuel. A fuelís flammability range refers to the percentage of the fuel, in
its gaseous state, to air to create a combustible or explosive mixture. This
varies with different flammable liquids. Gasoline has a flammability range
of 1.4 % to 7.6 %. This means gasoline will ignite when there is 1.4 parts
of gasoline mixed with 100 parts air. Thus, 1.4 % is known as the lower
flammable limit and 7.6 % is the upper flammable limit of the flammable
range. A product mixed with air below the low end of its flammable range is
too lean to burn. A flammable liquid that exceeds its upper flammable limit
is too rich to ignite. Ethylene oxide is extremely flammable. It has a
flammable range of 3.6 % to 100 %. This means it can burn even if there is
no air. The design of a Stanley burner is that of a shallow can. At
the top of the can is the burner grate that contains holes or slots. As the
fuel burns on top of the grate it draws in air and fuel from beneath the
grate. This causes a partial vacuum within the interior of the burner "can".
A pair of 1-1/2" diameter mixing tubes are inserted into the sidewalls of
the "can" under the grate. The mixing tubes draw in air to make up for the
air being drawn from the interior of the "can" to the topside of the grate
where burning is occurring. As the air is drawn into the mixing tubes
gaseous fuel under pressure is also injected at the proper fuel-air ratio
into the center of the mixing tubes. As the gaseous fuel and air travel the
length of the mixing tubes a through mixing occurs. As the mixture exits the
tubes it is drawn through the slots of the burner where it is burned above
the grate. Pictured at the left is a Cruban burner mounted to the
underside of a 20-horsepower Stanley boiler.
The design of a Stanley burner is that of a shallow can. At
the top of the can is the burner grate that contains holes or slots. As the
fuel burns on top of the grate it draws in air and fuel from beneath the
grate. This causes a partial vacuum within the interior of the burner "can".
A pair of 1-1/2" diameter mixing tubes are inserted into the sidewalls of
the "can" under the grate. The mixing tubes draw in air to make up for the
air being drawn from the interior of the "can" to the topside of the grate
where burning is occurring. As the air is drawn into the mixing tubes
gaseous fuel under pressure is also injected at the proper fuel-air ratio
into the center of the mixing tubes. As the gaseous fuel and air travel the
length of the mixing tubes a through mixing occurs. As the mixture exits the
tubes it is drawn through the slots of the burner where it is burned above
the grate. Pictured at the left is a Cruban burner mounted to the
underside of a 20-horsepower Stanley boiler. Where remembering the vapor density of gasoline and kerosene
is important is that the design of the Stanley burner provides ample space
within the burner for the fuel vapors to accumulate. This accumulation is
hazardous if an ignition source is available. Thus it is always important to
remember the potential for vapors to "pop" around a Stanley burner. Caution
must always be exercised when working around a Stanley burner.
Where remembering the vapor density of gasoline and kerosene
is important is that the design of the Stanley burner provides ample space
within the burner for the fuel vapors to accumulate. This accumulation is
hazardous if an ignition source is available. Thus it is always important to
remember the potential for vapors to "pop" around a Stanley burner. Caution
must always be exercised when working around a Stanley burner.
 Cruban offered a burner and pilot
light combination of their own design. Their burners were much heavier than
a Stanley being all cast construction. Cruban also built Stanley burner
bottoms using their Nichrome heat resisting lining. A Cruban grate is
similar in design to the Stanley slotted design. Crubanís aftermarket pilot
for use with a Stanley burner looked different in that they had a vertical
air intake. Click on the following link to view a series of
advertising cards describing the design features of the Cruban burner.
Also see the Steam Automatic article for information relating to the Cruban
Steam Automatic.
Cruban offered a burner and pilot
light combination of their own design. Their burners were much heavier than
a Stanley being all cast construction. Cruban also built Stanley burner
bottoms using their Nichrome heat resisting lining. A Cruban grate is
similar in design to the Stanley slotted design. Crubanís aftermarket pilot
for use with a Stanley burner looked different in that they had a vertical
air intake. Click on the following link to view a series of
advertising cards describing the design features of the Cruban burner.
Also see the Steam Automatic article for information relating to the Cruban
Steam Automatic. The
energy content of kerosene is between 130,000 BTUs and 135,000 BTUs per
gallon. The heat content of one gallon of kerosene roughly equals that
of 41 kilowatt-hours (kWh) of electricity, 137 cubic feet of natural gas,
1.5 gallons of propane, 17.5 pounds of air-dried wood, a gallon of diesel
fuel, or 10 pounds of coal. The Cruban burner in the experiment has
the capability to generate 500,859 BTUs per hour (135,000 BTUs per gallon of
kerosene x 3.71 gallons/hour). One (1) boiler horsepower is defined as
33,475 BTUs per hour thus the Cruban burner of the experiment is generating
about 15 boiler-horsepower (assuming total efficiency).
The
energy content of kerosene is between 130,000 BTUs and 135,000 BTUs per
gallon. The heat content of one gallon of kerosene roughly equals that
of 41 kilowatt-hours (kWh) of electricity, 137 cubic feet of natural gas,
1.5 gallons of propane, 17.5 pounds of air-dried wood, a gallon of diesel
fuel, or 10 pounds of coal. The Cruban burner in the experiment has
the capability to generate 500,859 BTUs per hour (135,000 BTUs per gallon of
kerosene x 3.71 gallons/hour). One (1) boiler horsepower is defined as
33,475 BTUs per hour thus the Cruban burner of the experiment is generating
about 15 boiler-horsepower (assuming total efficiency).